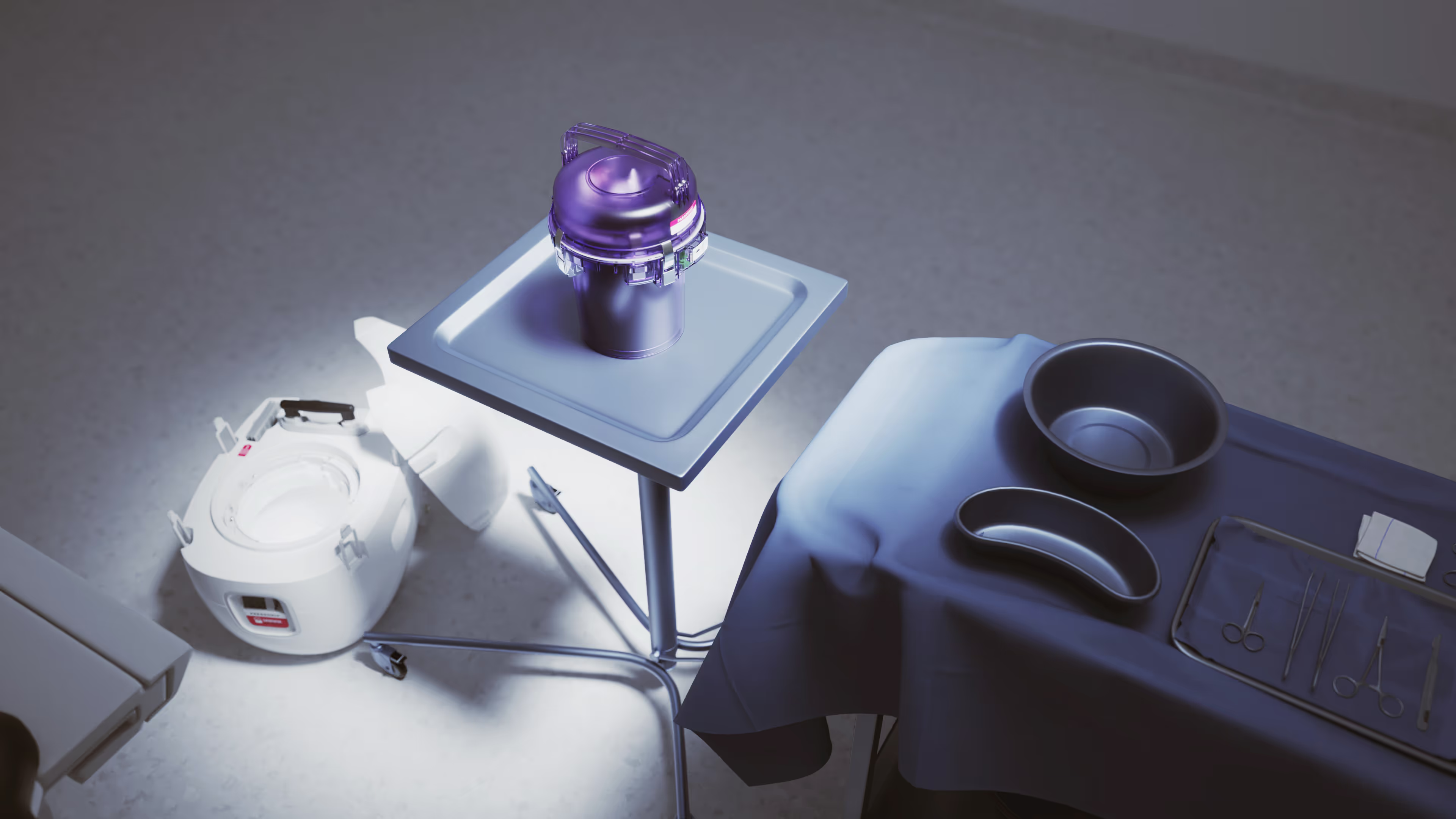
Traditionally, donor hearts destined for transplantation were placed into commercial-grade coolers filled with ice to transport it to the recipient. This method presents a multitude of uncontrolled variables that can impact the function of the heart post-transplant. Clinical evidence has shown that ice transport can expose the donor heart to freezing temperatures, leading to irreversible cellular damage.1
The Paragonix SherpaPak CTS is an Advanced Organ Preservation device specifically designed to standardize the variables of donor heart preservation, providing physicians with stable cooling technology driven by real-time data monitoring.
Caution: Federal (US) law restricts this device to sale by or an the order of a licensed health care practitioner. Please refer to Instructions for Use for full prescribing information. Cleared indication is for 4 hour use. Patents issued and pending. See legal page for more information.